Development of cell-omics framework
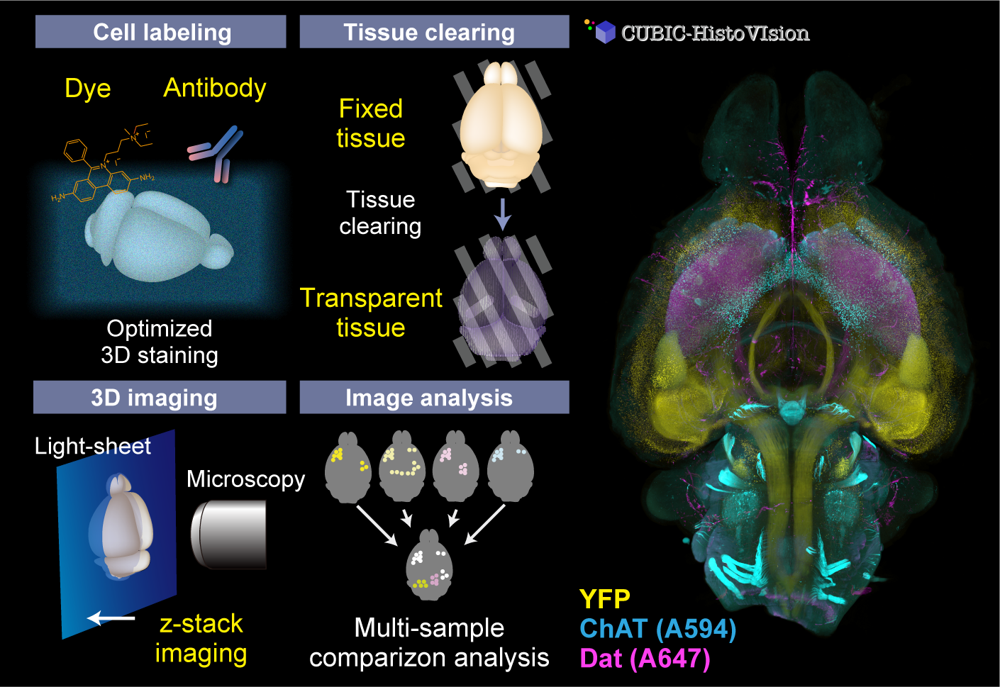
Organs and organisms consist of complex multicellular networks interacting with each other. However, there are a limited number of techniques for comprehensively observing and analyzing such cells and cell circuits. Our team continuously develops whole-organ and whole-body cell and cell circuit analysis (Cell-omics) technology to fulfill the gap. Our CUBIC (Clear, Unobstructed Brain/Body Imaging Cocktails and Computational analysis) workflow allows powerful tissue clearing, organ/body 3D imaging with light-sheet microscopy, and image informatics to extract biological information. To visualize cell and tissue architecture inside the organs and the body, we also invented CUBIC-HistoVIsion, a state-of-the-art 3D histological staining and imaging method. Together with our custom-built light-sheet imaging system and image analysis workflow, we successfully analyze the distribution, activity, and connection of multicellular circuits embedding whole organs and the whole body, expanding the possibility of multicelluar systems biology and 3D histopathology.
References:
Susaki et al. Whole-Brain Imaging with Single-Cell Resolution Using Chemical Cocktails and Computational Analysis. Cell (2014) *Highly cited article
Tainaka et al. Whole-Body Imaging with Single-Cell Resolution by Tissue Decolorization. Cell (2014)
Susaki et al. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat Protoc (2015)
Susaki et al. Whole-body and Whole-Organ Clearing and Imaging Techniques with Single-Cell Resolution: Toward Organism-Level Systems Biology in Mammals. Cell Chem Biol (review) (2016)
Nojima et al. CUBIC pathology: three-dimensional imaging for pathological diagnosis. Sci Rep (2017)
Tainaka et al. Chemical Landscape for Tissue Clearing based on Hydrophilic Reagents. Cell Rep (2018)
Susaki et al. Versatile whole-organ/body staining and imaging based on electrolyte-gel properties of biological tissues. Nat Commun (2020) *Top50 of 2020 Life & Biological sScience
Mano et al. CUBIC-Cloud provides an integrative computational framework toward community-driven whole-mouse-brain mapping. Cell Reports Methods (2020)
Susaki and Takasato. Perspective: Extending the Utility of Three-Dimensional Organoids by Tissue Clearing Technologies. Front Cell Dev Biol (2021)
Advanced biomedical imaging
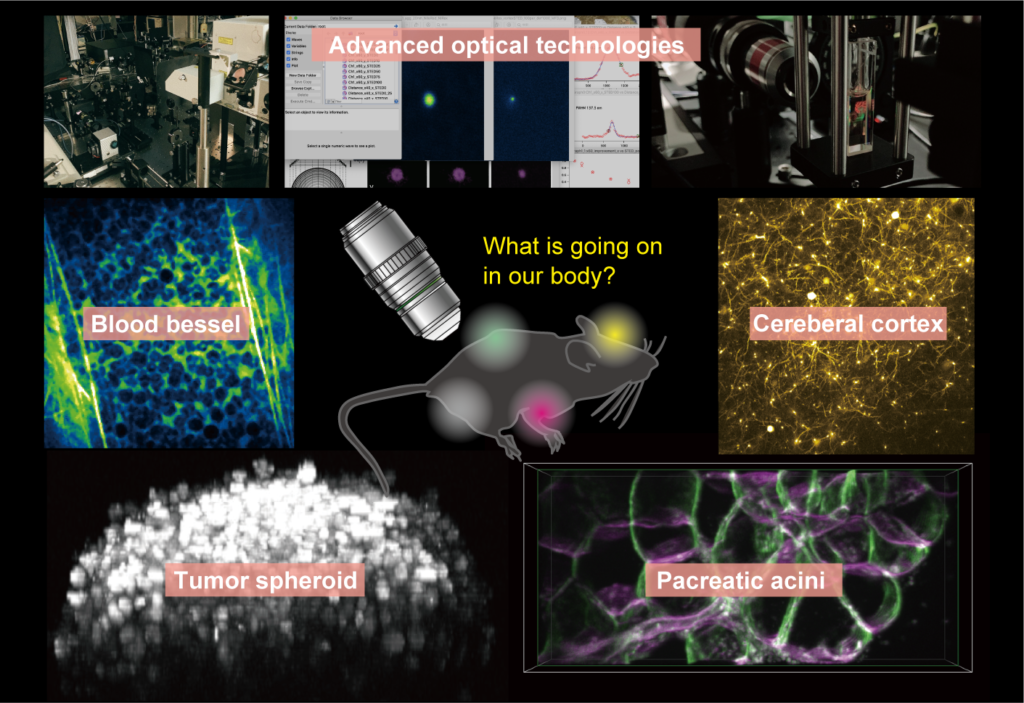
Fluorescent microscopy has become a powerful research tool for visualizing both static and dynamic processes in biological specimens. Recent cutting-edge technologies enable to understand biological nano/micro-structures, ultra-high-speed dynamics, multiple molecular/cellular interactions, and functional/structural networks across a large 3D volume. For unveiling our biological targets, we also started to develop novel systems in DBSB laboratory based on our expertise in non-linear microscopy, super-resolution microscopy, light-sheet microscopy, and some other optical technologies. Although simultaneous optimization of the performance characteristics of such technologies is near-impossible, identifications of trade-offs among them enable to develop truly practical tools. Our motivation for constructions of custom-built systems is not only breaking the record of bioimaging, more important is providing an essential piece of a pipeline for understanding biological phenomena and medical effects systematically.
References:
Otomo et al. Advanced easySTED microscopy based on two-photon excitation by electrical modulations of light pulse wavefronts. Biomed. Opt. Express (2018).
Goto et al., Real-Time Polarization-Resolved Imaging of Living Tissues Based on Two-Photon Excitation Spinning-Disk Confocal Microscopy. Front. Phys. (2019).
Yamaguchi et al. In vivo two-photon microscopic observation and ablation in deeper brain regions realized by modifications of excitation beam diameter and immersion liquid. PLoS ONE (2020).
Takahashi et al. PEO-CYTOP Fluoropolymer Nanosheets as a Novel Open-Skull Window for Imaging of the Living Mouse Brain. iScience (2020).
Wen et al. 3DeeCellTracker, a deep learning-based pipeline for segmenting and tracking cells in 3D time lapse images. eLife (2021).
Inflammation and host defense
We have been searching for effective therapeutic means for two of the major inflammatory diseases, sepsis and osteoarthritis (OA) by elucidating pathophysiology of the disorders and functions of protective molecules against them. Sepsis is a life-threatening organ dysfunction caused by a dysregulated host response to infection. Despite no robustly effective treatment is practical for sepsis, we revealed LL-37, a human-derived host-defense peptide, mitigates polybacterial murine sepsis. We found not only antimicrobial and lipopolysaccharide (LPS)-neutralizing activities of LL-37 but its various immunomodulatory functions such as inhibition of spontaneous neutrophil apoptosis and LPS-induced endothelial cell apoptosis, suppression of macrophage pyroptosis and pro-inflammatory cytokine production by macrophages, and induction of neutrophil extracellular traps (NETs). These functions are suggested being related to the sepsis-protective effect of LL-37. Furthermore, we demonstrated that LL-37 stimulates neutrophils to release extracellular vesicles (EVs), which possess antibacterial and anti-inflammatory potentials in the septic mice, thereby ameliorates sepsis pathophysiology. OA is a joint disease that involves synovial inflammation and chondrocyte degeneration. Glucosamine (GlcN) is a widely used dietary supplement with an expected effect of anti-OA. We reveled that GlcN suppresses the expression of pro-inflammatory genes in synovial cells. In addition, we clarified a role of GlcN in enhancing chondrocytes autophagy, which is considered a protective action by delaying OA progression.
References
Nagaoka et al., Cathelicidin family of antibacterial peptides CAP18 and CAP11 inhibit the expression of TNF-alpha by blocking the binding of LPS to CD14(+) cells. J Immunol (2001).
Suzuki et al., Human anti-microbial cathelicidin peptide LL-37 suppresses the LPS-induced apoptosis of endothelial cells. Int Immunol (2011).
Nagaoka et al., Modulation of neutrophil apoptosis by antimicrobial peptides. ISRN Microbiol (2012). https://doi: 10.5402/2012/345791.
Hu et al., Antimicrobial cathelicidin peptide LL-37 inhibits the pyroptosis of macrophages amd improvs the survival of polybacterial septic mice. Int Immunol (2016).
Someya et al., Glucosamine downregulates the IL-1β-Induced expression of proinflammatory cytokine genes in human synovial MH7A Cells by O-GlcNAc modification-dependent and -independent mechanisms. PLoS ONE (2016). https://doi.org/10.1371/journal.pone.0165158
Kumagai et al., Antimicrobial peptide LL-37 ameliorates a murine sepsis model via the induction of microvesicle release from neutrophils. Innate Immun (2020).
Cancer research
Under Construction